EFFECTS OF ENVIRONMENTAL HEAT ON CHILD,DR.DEV KUMAR JHA,MD. Child and Child Chest Specialist ,Delhi NCR
May 9th, 2024Please click on the link https://www.youtube.com/watch?v=X_C7Opms_fY

Please click on the link https://www.youtube.com/watch?v=X_C7Opms_fY
Please click on the link below
World Asthma Day is commemorated on every first Tuesday of May to increase awareness of asthma in public.
Today,7th May 2024 ,is World Asthma Day
There is a false perception among public that Asthma is a disease of adults and it does not occur in children.
The fact is that ,approximately 10-30 % children worldwide are suffering from Asthma and there are many hidden cases of Asthma in children ,where parents do not seek treatment from a qualified doctor who can diagnose Asthma in their children
The other problem is that ,it is very hard to accept the diagnosis of Asthma in children by their parents even if a qualified doctor tries to convince the diagnosis.
Ultimately ,child has to suffer the undiagnosed disease for years .
Dr Deo kumar Jha, MD, Pediatrician and Pulmonlogist,Delhi and NCR,Formery at AIIMS New Delhi
Asthma is the most common chronic respiratory disease in children.
It is both underdiagnosed and overdiagnosed in children
The standard treatment for childhood asthma is inhalational corticosteroid(ICS) in different doses according to the severity of asthma.
If the asthma is not controlled, on highest permissible doses of inhalational steroid,Long acting beta agonist(LABA) is added to control it, provided the technique of inhalation is correct,comorbidities have been addressed properly and allegen avoidence has been taken care of and adherence to treatment is good.
If it is not controlled on ICS+LABA,other add on options are LTRA(Monteleukast) and Tiotropium
If still the asthma is not controlled ,biologicals in the form of Omalizumab(IgE antagonist) and Meplozumab(IL5 antagonist) are given to control the asthma
Biologicals are costly with the disadvantages of adverse events and it is not widely available.
Asthma control is usually assessed by Asthma control test(ACT) ,Childhood asthma control test(CACT) and more easily by GINA guideline for control of asthma
Higher the ACT,CACT scores ,better is the control of asthma.
Researchers from the division of Pulmonology,department of Pediatrics,All India Institute of Medical Sciences,conducted an open label randomized control trial for a drug Azithromycin.Azithromycin is recommended drug by Global Initiative of Asthma(GINA) and British Thoracic Society(BTS) guideline for control of Asthma in adults.It improves spirometer parameter and reduces number of exacerbation of asthma in adults.There is no sufficient data for its use in children.
This the reason, researchers from Pediatric Pulmonology, division of the department of Pediatrcs AIIMS New Delhi, studied on 120 children between the age group of 5-15 years,mostly male(74% ) with poorly controlled asthma according to ACT and CACT.They divided these children into two groups.One group (n60) received Azithromycin in the dose of 10 mg/kg thrice weekly for 12 weeks along with standard treatment.The other group(n60) received only standard treatment.
The primary outcome was level of control of Asthma, according to ACT and CACT.Secondary outcomes were spirometry parameter,number of exacerbations,,Fractional excretion of NO(FeNO),throat swab culture positivity and adverse events
At the end of study period,the group who received Azithromycin along with standard care were having high ACT and CACT score (21.71 vs. 18.33; P < .001))indicating better asthma control.They also required less number of emergency visits due to asthma exacerbation and less use of oral or injectable steroids(0 vs. 1; P < .001).) ,higher number of good control of asthma by GINA guideline(41 vs. 10; P < .001).)
Spirometry parameters,throat swab culture ,FeNO reports and adverse events were not much different between two groups.
The benefits of Azithromycin was not different whether the child was suffering from eosinophilic or non eosinophilic asthma.
The study was published in CHEST.
CONCLUSION and BOTTOM LINE: Azithromycin in the dose of 10mg/kg,thrice weekly for 3 months may be added in treatment for children who could not achieve good control of asthma with standard therapy
REFERENCES:: Ghimire JJ, et al. Chest. 2022;doi:10.1016/j.chest.2022.02.025.
Assessment of hemodynamic conditions is most important in the management of critically ill patients
It is most important to pick up the condition of compensated shock and start treatment
Pediatric patients go into the stage of decompensated shock a bit late in comparasion to adult patients
Patients are managable in condition of decompesated shock if timely intervention is done.
Once the patient passes into irreversible shock it is very very difficult to revive and mortality is very high
How to recognise a patient clinically in a state of shock
STATE OF STABLE HEMODYNAMICS
Patient has clear consciousness
Periphery is warm and pink
Capillary refill time is 2 seconds or less
Pulse volume is good on palpation
Blood pressure is normal( between 5th to 95th centile for the age
Respiratory rate in the normal range for the age
Heart rate in the normal range for the age
Urine output normal for the age,1ml /kg/hour
STATE OF COMPENSATED SHOCK
Conscioussness inact
Periphery cool
Peripheral pulse ,low volume or thready
Capillary refill time more than 2 seconds
Blood pressure ,systolic is normal but diastolic is in rising trend,postural hypotension,narrow pulse pressure
Heart rate increased for the age
Respiratory rate increased for the age
Urine output reduced
STATE OF DECOMPENSATED SHOCK
Conscioussness-Restlessness or the patient is combative
Periphery on touch is cold and clammy
Capillary refill time is very prolonged like 5 seconds or more with or without mottling of skin
Peripheral pulse is very weak or may not be palpable at all even with great effort
Blood pressure -Hypotension,pulse pressure is 20 mm or less.Blood pressure may not be recordable
Heart rate-increased and in late stage decreased
Respiratory rate-Hyperpnea or Kussmaul breathing pattern(deep and sighy)
Urine output-oliguria or anuria
NORMAL RANGE of Respiratory rate
Premie-40-70/minute
0-3 months-30-60/minute
3-6 months-30-45/minute
6-12 months-25-40/minute
1-3 years-20-30/minute
3-6 years-20-25/minute
6-12 years-14-22/minute
>12 years 12-18/minute
NORMAL RANGE of heart rate- per minute
Premie 120-170
0-3 months 110-160
3-6 months 100-150
6-12 months 90-130
1-3 years 80-125
3-6 years 70-115
6-12 years 60-100
>12 years 60-100
HYPOTENSION is called when systolic blood pressure is below
60mm of Hg in NEW BORN,
70 mm of Hg between the age of 1 month to 1 year
70 mmHg+age in years multiplied by 2 ,between the age of 1- 10 years
90 mm Hg above the age of 10 years
Hypotension is also called when mean arterial pressure (MAP)is below 40+age in years multiplied by 1.5
MANAGEMENT:Management should start at the earliest, at the stage of compensated shock
First attention should be on airway and breathing and oxygen should be given if required to keep SPo2 95% and above
Life saving -for the circulation to be maintained, is fluid therapy
20 ml/kg of N/S or R/L shuold be given over 5-15 minutes and it should be pushed.It can be repeated twice if hydration,circulation and perfusion is not adequate.
In the settings of obvious fluid loss like diarrhoea ,vomiting or hemorrhage ,repeated fluid administration should be done till the signs of fluid overload develop, in the form of tachycardia,bilateral deep inspiratory crackles over subscapular region,liver enlargement,engorgement of jugular vein or signs of pulmonary edema on chest X-Ray
R/L shuold not be used in case of a history of repeated vomiting
IV bolus should be repeated ,only when there is sign of improvement clinically and no sign of fluid overload.
Aggressive fluid therapy may be harmful and should not be given in certain situations like shock in the settings of severe acute malnutrition,severe anemia,compensated shock with high fever with no dehydration or obvious fluid loss(Dengue fever),cardiogenic shock(ductal dependent congenital heart disease in newborn),obstructive shock(tension pneumothorax,cardiac temponade,)
SIGHNS OF CARDIOGENIC SHOCK-Tachycardia,engorged jugular vein,bilateral deep inspiratory crackles over subscapular regions,gallop rhythm,liver enlargement,signs of pulmonary edema on chest X-Ray.
In these cicumstances,crystalloids(N/S or R/L) should be given in the dose of 5-10 ml per kg over 15-30 minutes once then switch over to vasopressors
In case of poor response or no response to fluid therapy,swith over to vasopressures without delay.
If the periphery is cold,give DOPAMINE/EPINEPHRINE
If the perphery is warm give NORADRENALINE
In case of myocardial dysfuntion with maintained blood pressure,give DOBUTAMINE
In case of myocardial dysfuntion with increased peripheral resistance,use MILRINONE
Easy preparation and administration of vasopressures
Dopamine/Dobutamine 6 mg/kg-dilute in 100 ml of D5-1 ml/hour of this will deliver 1 mcg/kg/minute=Dose is 5-20 mcg/kg/minute
EPINEPHRINE0.6mg/kg of body weight,dilute in 100 ml of D5-1ml/hour will deliver 0.1mcg/kg/minute=Dose 0.05 to 0.2mcg/kg/minute,in severe cases upto 1mcg/kg/minute
Norepinephrine 0.6 mg/kg,dilute in 100 ml D5,1ml/hour will deliver 0.1mcg/kg/minute=Dose 0.1-1mcg/kg/hour
REFERENCES1.;Harriet Lane 21 edition
2. CDC guideline on management of shock in children
3.Uptodate-management of shock in children,2021
In the period of october 2021,both Dengue fever and Multi system inflammatory syndrome in children(MIS-C) are being seen in children in Delhi,India
Clinical and laboratory features are overlapping for both these diseases
It is important to differentiate between these as management entirely differs for both
In case of Dengue fever the cornerstone of management is aggressive fluid therapy with crystalloid and colloid with ionotrops if fluid therapy does not work and platelet transfusion if needed.
In cases of MIS-C, the cornerstone of management is steroids and IVIG(Immunoglobulins).Aggressive fluid management may be detrimental in cases of shock with cardiac dysfunction.
Fever are common in both but swellings of feet and hands,diarrhoea,conjuntival injections and altered sensorium along with the laboratory findings of hyperinflammation like highly raised CRP,Leukocytosis,raised D-Dimer are pointers towards MIS-C .In this situations,anti COVID antibody should be done and if positive ,confirms the diagnosis of MIS-C
If fever is associated with vomiting ,erythmatous rashes,myalgia along with the laboratory findings of leucopenia ,severe thrombocytopenia,hemoconcentration , raised serum ferritin level,it points towards the diagnosis of Dengue fever and NS1 antigen and or anti Den IgM should be done which when positive confirms the diagnosis of Dengue fever
In comparision to MIS-C,serum Ferritin level is higher in Dengue fever
References:
1. Ahmed M, Advani S, Moreira A, et al. Multisystem
inflammatory syndrome in children: A systematic review. E
Clin Med. 2020;26:100527.
2.Mishra S, Ramanathan R, Agarwalla SK. Clinical profile of
dengue fever in children: A study from southern Odisha,
India. Scientifica (Cairo). 2016;2016:6391594
3.Indian Pediatrics,volume 58,15 October,2021
The most common chronic respiratory disease from which children all over the world suffer is ASTHMA.
Please click to know more for the better care of asthmatic children.
Please click below to watch DR.D.K.JHA discussing management of asthma in children in accordance with GINA guideline.
Please click below to watch DR.D.K JHA,talking invasive Pediatric Mechanical ventilation in a very simple and enjoying way

Coronavirus (SARS-Cov 2) infections causing Coronavirus disease(Covid 19) have killed many people worldwide since its spread starting from November 2019.
Vaccines are now available worldwide to protect the world populations by synthesising antibody in human body which fights and kills Coronavirus when it enters into the body.
Vaccines are not recommended currently for pregnant women as well as for infants and children.
In such scenario, if it is known that coronavirus infections stimulate the production of antibody against it and it is transferred from mothers to newborn infants to protect them,it will be gift to infants by nature.
In childrens hospital Philadelphia,1714 women were studied who delivered between April 9 to August 8,2020.
Immunoglobulin G(IgG) were measured from the serum of mothers and cord blood of their newborns in 1471 cases
SARS-CoV 2 antibody IgG were detected in 83 women,that is in 6% women.This IgG is known to cross the placenta.
Among these 83 women,72 newborns were detected to have IgG,that is in 87% cases.
No antibody was detected in newborns of seronegative mothers.
In 11 newborns of seropositive mothers,no antibody was detected.Among them,5 mothers were having only IgM ,which does not cross the placenta and 6 mothers were having very low titre of IgG.
So, it is very important study,which showed the presence of protective antibody in newborns of mothers who were infected with Coronvirus and produced protective antibody for themselves as well as for their newborn infants.
REFERENCES:JAMA Pediatrics,Published online on January 29,2021
Tuberculosis is very very old disease of human .
There has been many research in the field of diagnosis and treatment of tuberculosis.
Inspite of all the efforts worldwide,it is still the killer disease due to various reasons responsible for the emergence of drug resistant TB (Tuberculosis).
Chief responsible factors for the increased incidence of tuberculosis are poverty leading to undernutrition, and HIV infection.
Main causes of emergence of drug resistance is not completing the prescribed regimen of treatment and high burden of tuberculosis.
TERMINOLOGIES BEING USED:
Monoresistant tuberculosis-Resistance of tuberculosis to any first line drug-Rifampicin,Isoniazid,pyrazinamide,ethambutol
Polyresistant tuberculosis: Resistance of tuberculosis to more than one drug but not to both Rifampicin and Isoniazid
Multi drug resistant(MDR) tuberculosis: Resistance to both Rifampicin and Isoniazid with or without resistance to other drugs
Pre-extensively drug resistant(PRE-XDR) tuberculosis:Resistance to both Rifampicin and isoniazid with resistance to either fluoroquinilones or second line injectables but not to both fluoroquinolones and second line injectables(SLI)
Extensively drug resistant tuberculosis(XDR):Resistance to Rifampicin,Isoniazid,fluoroquinolones and second line injectables(SLI)
RR-TB:Resistance to Rifampicin with or without resistance to other antituberculous drugs
SECOND LINE INJECTABLES(SLI):Amikacin,Kanamycin and capreomycin
PRIMARY RESISTANCE:When a child or adult becomes infected with drug resistant strain of Mycobacterium tuberculosis
SECONDARY/ACQUIRED RESISTANCE:This is more common.The individual is infected with drug sensitive strain of Mycobacterium tuberculosis but it becomes drug resistant during treatment due to selection of resistant mutant strain.The cause of such resistance is incopmlete or suboptimal treatmen
TERMS USED IN DIAGNOSTIC PROCESSES
C-Tb skin test;This is a new test for the detection of tuberculosis infection.In this test ESAT6/CFP10 antigens are used.It is done in the same way as Tuberculin skin test(TST).Antigen is injected intradermally on the forearm and reaction is read after 48-72 hours with the ball pen-scale method.An induration of 5mm is taken as positive irrespective of age,BCG status and whether with HIV or non HIV.The sensitivity is comparable to TST(MANTOUX TEST) and IGRA(QUANTIFERON GOLD)
IGRA(QUANTIFERON TB GOLD IN TUBE TEST;QFT-GIT: AND T-SPOT TB TEST:T-SPOT):This test is based on the principle of white blood cells of individuals infected with mycobacterium release interferon gamma when mixed with antigens derived from Mycobacterium.In this test whole blood is taken from individual and then mixed to ESAT6/CFP10 antigens and result is available within 24 hours.It does not differentiate between active and latent Tb.This test is not affected by BCG vaccination and is specific for Mycobacterium tuberculosis but not reliable below 5 years of age.
TESTS TO DETECT MYCOBACTERIUM:
LAMP;Loop mediated isothermal amplification test is 15% more sensitive than Zeil Neilson microscopy(smear microscopy) which is most widely used traditional test to detect Mycobacterium in smear preparation of sample in the form of sputum or gastric aspirate. It is temperature independent test ,done manually for amplification of DNA and can be read by naked eye with ultraviolate light.The report is available within one hour.WHO has recommended it as an alternative to ZN microscopy as it can be used in periphery
LED-FM:Light emitting diode fluorescent microscopy is 10 % more sensitive than ZN microscopy.With proper training it can be used in periphery although its specificity is less.WHO has recommended it as an alternative to ZN microscopy.According to WHO policy paper its sensitivity is 86.3%
CBNAAT :Cartridge based nucleic acid amplification test is based on polymerase chain reaction for the ampilification of DNA.Report is available within 2 hours.It can detect live as well as dead tuberculous bacilli ,so it can not be a replacement for smear microscopy and culture based drug sensitivity test for folllow up.It is also known as GenXpert /Rif test.It also detects Rifampicin resistance.Its sensitivity is 89% and specificity is 99%
GenXpert ultra(CBNAAT ULTRA):It is an advance version of GenXpert which is ultrasensitive with main difference from GenXpert is ,it can detect Mycobacterium from sputum even if the number of bacilli per ml is as low as 16,whereas in GenXpert ,the number of bacilli should be 131/ml for detection
TRUENAT;It has been developed in India by Molbio Diagnostics Pvt.Ltd.Goa.Its sensitivity and specificity to detect Mycobacteria and Rifampicin resistance is similar to CBNAAT/GenXpert test.But it requires 0.5 ml of sample as compared to CBNAAT which requires 1 ml.It is battery operated and not fully automated so it does not require continuous power supply and can be used in periphery

LPA-Line probe assay is based on polymerase chain reaction with reverse hybridization technique.First line assay detects resistance to isoniazid while second line LPA detects resistance to Fluoroqinolones and SLI.Report is available within 24-48 hours.According to recent RNTCP guideline,if Rifampicin resitance is detected on CBNAAT,second sample is sent to detect isoniazid resistance by LPA.If isoniazid resistance is detected,second line LPA is done for Fluoroquinolones and SLI.If Rifampicin sensitivity is detected on CBNAAT,sample is sent for LPA to detect isoniazid resistance.
According to WHO,END TB Programme,all patients should be subjected to DST(Drug sensitivity test) and the reference standard for this test is either liquid or solid culture.The report becomes available in 12 weeks.
To meet the requirement of universal DST as recommended by WHO,rapid tests are being developed as-NEXT GENERATION SEQUENCING(NGS).It is a rapid molecular test to detect mutations responsible for drug resistance.These are of 3 types
Targeted NGS-it sequences the specific point on gene of Mycobacterium tuberculosis
Whole genome sequencing(WGS)It sequences the whole genome ,so it is better than TNGS.
Pyrosequencing-it is method of sequencing by synthesis.
DRUGS TO TREAT RESISTANT TUBERCULOSIS:
GROUP-A-Levofloxacin or Moxifloxacin,Bedaquiline,Linezolid
GROUP-B-Clofazimine,Cycloserine or Teridizone
GROUP-C-Ethambutol,Delamanid,Pyrazinamide,Imipenam-cilastin or Meropenam,Amikacin or Streptomycin,Ethionamide or Prothionamide,Para-Aminosalicylic acid(PAS)
There are TWO regimens for the treatment of drug resistant tuberculosis,Long course and Short course
Long course is for 18-20 months-According to WHO 2019 guideline, 2 drugs from Group A except Bedaquiline in children,1-2 drug from Group B along with Delamanid must be chosen and the list of at least 5 drugs is completed from Group C.After 6 months of continuation phase,Delamanid is withdrawn and at least 4 drugs should be continued for the rest of the period of treatment..
DELAMANID CAN BE GIVEN TO CHILDREN ABOVE 3 YEARS OF AGE
SHORT COURSE REGIMEN:It is given for a period of 9-12 months.Usually the intensive phase is of 4-6 months consisting of Moxifloxacin,high dose isoniazid,ethambutol,,pyrazinamide,clofazimine,ethionamide or prothionamid,Kanamycin or Amikacin(7 drugs) followed by a fixed period of 5 months of Moxifloxacin,clofazimine,pyrazinamide and ethambutol(4 drugs)
Drug resistance to Fluoroquinolones and second line injectables should be ruled out before initiating short course treatment.
Now a days it is being emphasised and WHO in June 2020 has recommended ,all oral drug regimen where injectables shuold be replaced by Bedaquiline.FDA has approved Bedaquiline above 12 years of age but in India it has been approved above 18 years in accordance to RNTCP guideline.
NOTE:High dose isoniazid- dose is 15-20 mg/kg/day-it can cause optic and peripheral neuritis,ANA positivity,agranulocytosis,vasculitis and thrombocytopenia.
Linezolid-Dose 15 mg /kg od for wt<15 kg and 10-12 mg/kg od,for >15kg.It causes Myelosuppression,peripheral and optic neuritis and lactic acidosis.It penetrates CNS well
Ethionamide/Prothionamide causes hypothyroidism
EPTB and CNS Tb should be treated with longer regimen
REFERENCES:
TB facts.GenXpert Test-TB diagnosis,TB resistance testing,CBNAAT.2018.Available at:http://www.tbfacts.org/genexpert/Accessed
2019
World Health Organisation(WHO).The use of next generation sequencing technologies for The detection of mutations Associated with drug resistance in Mycobacterium toberculosis Complex;Technical guide.Available at http://apps.who.int/iris/handle/10665/27443.Accessesd2019.
World Health Organisation.WHO consolidated guideline on Drug Resistasnt tuberculosis treatment 2019?Available at :https:apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf.Accessed 2019
RSV(Respiratory syncytial virus) is the most common cause of bronchiolitis in children.
Bronchiolitis is usually mild but in infants between 3-6 months,it may become serious and sometimes life threatening.
It usually becomes serious in preterm infants and infants already having cardiac disease with significant shunt,infants having CLD(Chronic lung disease,previously known as BPD) and infants with primary immune deficiency.
There is no specific treatment other than Rivaverin which may or may not work.
So, the mainstay of treatment is only supportive and it has high morbidity and mortality.
In such situations,a new drug Sisunatovir may be life saving for many infants.
In a research ,66 adults were inoculated with RSV,then they were given treatment with this new drug Sisunatovir.It resulted in significant reduction in clinical symptoms with significant reduction in viral load,with no significant adverse effects.The new drug was well tolerated and there was no resistane to this new drug.It was Phase 2a study.
Sisunatovir binds to the surface protein F, of RSV and inhibits its replication.It is a fusion inhibitor which is administered orally.
REFERENCES:ReViral announces FDA Fast Track designation granted to sisunatovir for the treatment of serious respiratory syncytial virus infection. https://www.businesswire.com/news/home/20200804005065/en/ReViral-Announces-FDA-Fast-Track-Designation-Granted. Accessed August 4, 2020
Computed tomography of chest(Chest CT) has become an essential imaging modality to diagnose many chest conditions including some complicated Pneumonia.
As CT chest is time consuming,so children below 3 years should better be sedated for proper examination of chest by CT.
Ground glass opacity (GGO) may be seen in different chest conditions and its location may give some clue to the diagnosis.
In the time of widespread occurance of COVID 19 ,CT chest may be useful to point towards diagnosis when the serological tests are negative.
In COVID 19 Pneumonia typical findings on chest CT is bilateral ground glass opacity located peripherally in the subpleural regions and at lower lobes of lung.Later on there may be crazy pavy changes with architectural damage ,perilobular opacities superimposed upon ground glass lesions.
There may be consolidation in the lower lobes of the lung located in subpleural regions suggestive of COVID19 Pneumonia.
Atypical findings in COVID19Pneumonia may be ground glass opacity on the upper lobes of lung,peribronchovascular regions and,there may be cavitation,lymphadenopathy and pleural thickening.
Ground glass opacities are also seen in other viral pneumonia.It is seen in upto 75%cases of Adenovirus Pneumonia and more than 75%cases of cytomegalovirus(CMV) Pneumonia.It is also seen in approximately 25% cases of Herpes simplex Pneumonia.
It is also seen in Pneumonia due to Pneumocystis carini but in this case it is mainly located on the upper lobes.
GGO may be observed in interstitial lung diseases(ILD) but the pattern of distribution may differentiate it from infective origin.
GGO may be seen in lung injury caused by electronic cigarrette smokes,eosinophilic pneumonia,hypersensitivity Pneumonia,Pulmonary alveolar proteinosis,diffuse alveolar hemorrhage,and pulmonary edema.
Bacterial Pneumonia can be differentiated by the distribution of opacities in the focal ,segmental and lobar regions not predominantly in the lower lobes.Other findings also differentiate it from viral pneumonia, like cavitation,lymphadenopathy and lung abscess
REFERENCES;Cite this: Broad Differential Diagnosis of Chest CT Ground-Glass Opacities – Medscape – Jul 16, 2020.
Coronavirus 19 is a potential lethal virus causing Coronavirus disease 2019.
This disease has made realisation of its presence all over the world .
All age groups are being infected with this disease with varying morbidities and mortalities.
The disease has disrupted the educational and economic activities,all over the world.Schools are closed for a long time all over the world in the fear of spread of the disease among children and then in the household.
Researchers have studied 4130 cases through hospital surveillance network between 10,March 2020 to 10,April 2020.Among them 40 cases were children below 16 years of age.Household and parents were called for contact tracing .
It was observed that,in 79% of cases, the source were an adult in the household who were either diseased or infected prior to the infection in children.
In only 8% cases,children were primarily infected who then infected adults.
It was then concluded that,children, not only suffer from mild form of the disease in majority of cases,but they are also not the source of infection to othe children or adults in most of the cases.
The study was published in PEDIATRICS,the official journal of American Academy of Pediatrics.
In conclusion,there will be very little benefit from closing the schools, but it can adversely affects the academy of growing children.
REFERENCES:Pediatrics. 2020;146:e20201576, e2020004879
Famotidine is H2 receptor antagonist which is out of fashion now,which has been widely used to treat gastritis in children as well as in adults.
According to a case series of 10 patients of COVID19, Famotidine was self administered at home as it is a over the counter drug,means anyone can purchase it from medical store without the prescription of a doctor.
The dose used was 80 mg three times daily for a median period of 11 days.
Patients were interviewed on telephone regarding ,demography,risk factors,temperature,oxygen saturation and general well being apart from commom symptoms related to COVID 19.
According to the National institute of health protocol,longitudinal severity scores of 5 symptoms were collected-headache,cough,fatigue,shortness of breath and ansomnia.
The demography and risk factors were wide in the patients.Symptomps started to improve after 24-48 hours of initiation of therapy and the patients came to premorbid condition 14 days after treatment.
Particularly airway related symptoms like cough and shortness of breath improved faster than general symptom like fatigue
The mechanism of action of this drug Famotidine has not been established,which needs further study.
REFERENCES:Janowitz T, Gablenz E, Pattinson D, et al. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series [published online June 4, 2020]. Gut. doi:10.1136/gutjnl-2020-321852
Asthma is a leading cause of respiratory morbidity all over the world whether it be adult or children.In cough variant asthma,patients have only symptom of troublesome cough for a long period.They do not complaint of tightness of chest,difficulty in breathing,or wheezing any time in the course of their illness.On CHEST auscultation,there is no adeventitious sound in the form of wheeze.This type of cough presents a difficulty in front of treating physician for the accurate diagnosis.It may be asthma or other diagnosis which needs meticulous investigations.It is also difficult to convince to patients or parents that it may be asthma,because there is a common belief among patients and parents that asthma means difficulty in breathing.On the other hand,spirometry,which is a goldstandard diagnostic test for asthma, done on such type of asthma patients are usually normal.
FeNO(Fractional excretion of nitric oxide) is measured by a portable machine,which is hand held and subject is asked to exhale through the mouth piece connected to a hand held device.The measurement is in part per billion(ppb).The normal and abnormal levels have been validated in adults,not in children.But its level when it is high,well correlates with eosinophilic inflammation of airways in children and it can be performed easily in school going children.
In a study ,32 patients with an average age of 42.5 years were included.All had only cough for a long period(chronic cough),normal blood eosinophil count,normal chest X-Ray,normal spirometry results but abnormal ACT(Asthma control test) and positive skin prick test for environmental antigens.9 healthy persons were included for control with the mean age of 47 years.FeNO measurements were taken with the help of FeNO analyser.
FeNO were significantly elevated with the mean value of 64.4ppb in 91%(n29) of patients.The normal cut off value for adults is 25ppb.In healthy controls,the mean value measured was 16ppb.
REFERENCES:Nsouli T, Diliberto N, Nsouli S, Zamora S, Nsouli A, Bellanti J. Lack of concordance between FeNO and spirometry in patients with chronic cough. Presented at: American College of Allergy, Asthma, & Immunology Annual Scientific Meeting 2019; November 7-11, 2019; Houston, TX. Abstract A202
Sometimes ,it becomes difficult to differentiate asthma,COPD and asthma-COPD overlap
It is easy to take sputum sample in adult, but in children it is difficult
So,any sample can be used depending upon the age of patient,if both correlates well
Researchers from Japan retrospectively,evaluated 195 patients with Asthma(n95),COPD(n61) and asthma COPD overlap(n39) between 2015 to 2017
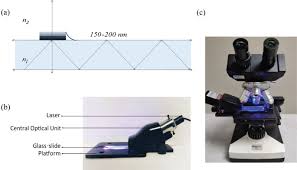
Sputum sample was centrifuged at 500g for 5 minutes,then transferred to a glass slide.It was then crushed with a rotatory motion with another glass slide.Cell counts were done after proper staining.
To assess whether the sputum eosinophil counts-/>3% correlates with blood eosinophil count,researchers constructed ROC(Receivers operative curve)
Patients with Asthma and asthma COPD overlap had significantly higher eosinophil count and lower neutrophil counts during stable period as compared to patients with COPD . But it was not so during exacerbations.
MEAN eosinophil and neutrophil counts: asthma, 17.4% and 78.8%, respectively; COPD, 1.8% and 95.6%, respectively; and asthma-COPD overlap, 11.8% and 84.2%, respectively).It was during stable period
But,during the periods of exacerbation, following was the results
MEAN eosinophil and neutrophil counts: asthma, 15.0% and 83.3%, respectively; COPD, 4.8% and 86.4%, respectively; and asthma-COPD overlap 17.7% and 79.9% respectively.
There was significant correlations between blood and sputum eosinophil percentage among all categories of patients both during stable and during the period of exacerbations
It was clear from the analysis of ROC curve, that the blood eosinophil can predict sputum eosinophil count3% during stable period as well as during the period of exacerbations.Following is the results.
During stable period (AUC, 0.75 [95% CI, 0.66-0.84]; cutoff, 235 µL; sensitivity, 75.4% and specificity, 71.3%) and during the exacerbation period (AUC, 0.80 [95% CI, 0.66-0.94]; cutoff, 351 µL; sensitivity, 61.1% and specificity, 97.1%).
So we can take either sample according to feasibility and during the stable period ,we can diffferentiate between asthma,COPD and asthma COPD overlap
REFERENCES:
Tamura K, Shirai T, Hirai K, et al. Differentiation of asthma, COPD, and asthma-COPD overlap via a simplified sputum cell count method. Presented at: CHEST Annual Meeting 2019; October 19-23, 2019; New Orleans, LA. Abstract 464.
Pediatric tuberculosis is a burden to society and nation .
It is prevalent in every society and every nation.
It spreads by aerosols which comes in air after coughing by a diseased person and then inhaled by healthy person .
In children ,it is mostly contracted by a diseased adult suffering from pulmonary tuberculosis .
Lifetime risk for an infected child to become diseased is 10%.
CBNAAT-cartridge based nucleic acid amplification test, also known as GeneXpert test is now investigation of choice to detect Mycobacterium tuberculosis in children suspected to be suffering from tuberculosis .
The sensitivity of this test in sputum smear positive case is 98% and specificity is 99% but in smear negative and culture positive cases its sensitivity is only 72% but specificity is 99%
In GA(gastric aspirate sample ) the sensitivity is only 68% in culture positive sample and specificity is 99%.
Presently it is done on sputum, gastric aspirate ,CSF ,pleural fluid ,lymph done aspirate ,ascitic fluid,synovial fluid but not on blood .
In lymph node aspirate,the positivity is 35%.
In some children,in which induced sputum and gastric aspirate are negative ,BALf,bronchoalveolar lavage fluid obtained by bronchoscopy has been found to be positive.
The sensitivity is low in Synovial fluid,pericardial fluid,ascitic fluid and very low in pleural fluid.
SO ,NEGATIVE TEST RESULT OF CBNAAT/GeneXpert TEST DOES NOT RULE OUT TUBERCULOSIS
Only one sample is needed and if unable to send the sample to lab immediately, it can be stored safely in refrigerator for 7 days but should not be freezed .
It is a real time PCR test and gives result in 2 hours.
It detects Mycobacterium tuberculosis as well as its resistance to Rifampicin.
If resistance to Rifampicin is detected and there is no suspicion of resistant tuberculosis clinically,then a fresh second sample is sent.
In second sample, if there is sensitivity to Rifampicin, it is labelled as Drug sensitive TB.
In GeneXpert Ultra test,second sample is not required.
The yeild is high in this test if there is chest X-ray findings suggestive of tuberculosis.
In case of only clinical suspicion with no radiological findings,the sensitivity is approximately 10%
SO,FOR THE HIGH YIELD,THIS TEST SHOULD BE SENT WHEN THERE IS SUSPICIOUS LESION ON CHEST X-RAY
In case of pleural effusion,the highest yield is from the examination of pleural biopsy which is positive in 80% cases.
The culture of pleural fluid is positive in only 10% of cases.
Other recommended tests are LPA-Line probe assay and LAMP-Loop mediated isothermal amplification.
These tests (CBNAAT,LPA annd LAMP)are called WRDT-WHO recommended rapid detection test.
The Gold standard diagnostic test is now, liquid culture in the form of MGIT-Mycobacterium growth indicator tube culture which gives result in 3 weeks.Previously it was solid culture.
Culture is positive in 1/3 to 1/2 cases of Tuberculosis.
FUTURE PROSPECT: CBNAAT has been used to detect Mycobacterium tuberculosis in stool sample in children.Its sensitivity in one study in children and persons with HIV has been found to be over 80% and specificity over 95% when compared to respiratory sample.
After multicentre study,it may become the preferred sample for children in which respiratory sample is difficult to obtain.
TREATMENT:
Category II (CAT II) Treatment comprising of 2HRZES+1HRZE+5HRE has been completely withdrawn now.
There is only one category now, for all patiens ,comprising of 2HRZE+4HRE,FIRST LINE ATT
For newly diagnosed cases,whether smear positive or smear negative this treatment should be completed for 6 months.
All patients,who have not taken ATT previously or have taken it for less than 4 weeks are labelled as New Case
In cases of neurotuberculosis or spinal tuberculosis,the continuation phase comprising of HRE should be extended for 8 months.
In cases of relapse,defaulters, retreatment,treatment after failure, and any contact with resistance tuberculosis,the sample should be sent for DST-Drug sensitivity test, while the treatment started as 2HRZE+4HRE.
If the result comes as sensitive to first line medications,the treatment should be completed with this regimen only
If resistance comes to any drug, then the second line drugs should be started according to the sensitivity pattern.
Second line drugs are less potent and should be given for prolonged time.
Two highly potent drugs Dalaminid and Bedaquilline are now recommended for treatment of children with resistant tuberculosis.
Bedaquilline is recommended for children 6 years and above.
Dalaminid is recommended for children 3 years and above.
These two drugs are available at selected centres in India
In cases of LTBI -Latent tuberculosis bacillus infections,in which only Tuberculin sensitivity test or IGRA is positive but there is no clinical symptom and sign or any lesion in any organ suggestive of tuberculosis,no treatment is given in India.
In Western countries,the current recommendation is to treat LTBI with 12 Doses of HP-3HP-(3 months of HP)
Previously it was recommended for adults,but now it is recommended in children also
In such cases(LTBI),weekly doses of Rifapentine and isoniazid is given for 12 weeks.
Currently,it is not recommended for children below 2 years of age.
All children receiving isoniazid should be given daily dose of 10 mg of pyridoxine.
Definite indications of steroid along with ATT are TBM,Pericarditis,Addisons disease,Miliary TB with alveolocapillary block and TB uveitis.
Steroid can be given in endobronchial tuberculosis,pleurisy with severe distress,bronchial compression,mediatinal compression syndrome,laryngeal TB,and TB-IRIS(Immune reconstitution inflammatory sundrome).
Evidence is not sufficient for tuberculoma.
Prednisolone 1-2 mg/kg/day or dexamethasone 0.6mg/kg/day or any steroid in equivalent doses ,should be given for 4 weeks then tapered over next 4 weeks.
REFERENCES:
RNTCP Updated Pediatric TB Guidelines 2019 developed by Revised National TuberculosisControl Programme and Indian Academy of Pediatrics.
Guidance document draft as on 04.02.2019,Central TB division,Ministry of Health and Family Welfare,New Delhi India
CDC:Treatment Regimen for Latent TB infection
CDC:The 12 dose Regimen forLatent Tb infecvtion Treatment:Fact Sheet for clinicians
Eur Respir J 2019 53:1801832; published ahead of print 2018,
doi:10.1183/13993003.01832-2018
;
Asthma is the most common chronic disorder in children all over the world.
Its incidence and prevalence has increased in last decades even in the presence of more advance diagnostic modalities and more sophisticated treatments available now a days.
The cornerstone of controller medication for asthma is inhalational steroid.
Injectable and or oral steroid is the principal agent for the treatment,and becomes life saving, when a child lends up in emergency department with acute severe exacerbation of asthma.
Few children with asthma do not respond to steroid therapy and the physicians lend up in problem when they give steroid, spend time and then do not see any improvement in the child.
In such situations,if we know which child will not respond to steroids,will be very helpful.
In a research study,192 asthmatic children who were on inhalation steroid treatment and 130 healthy were included.
Serum level of OX40L was estimated in all children.Its level was correlated with clinical characteristics of asthma and the response of steroid treatment.Serum levels of eosinophils,Nutrophils,IgE,Interleukin 6 and TSLP(Thymic stromal lymphopoietin) were estimated in all children.
There was a positive correlation between serum levels OX40L and serum levels of Eosinophils,Nutrophils,IgE,Interleukin 6 and TSLP which are known serum markers of asthma and its severity.
There was a significant high level of serum OX40L in asthmatic children in comparision to healthy children and the level of serum 0X40L was much higher in steroid resistant asthma(SRA) in comparision to Steroid sensitive asthma(SSA)
There was a negative correlation between serum level of 0X40L and Asthma score test(AST) and FEV1 which are measured to assess the severity of asthma at regular intervals.
So,the high level of serum 0X40L has 2 meanings,firstly, it indicates more severe asthma and secondly very high level may suggest the asthma is unresponsive to steroid treatment.
In future , the agent targetting this 0XL40 is required to customise the treatment of asthma.
REFERENCES:Elevated serum OX40L is a biomarker for identifying corticosteroid resistance in pediatric asthmatic patients
BMC Pulmonary Medicine — Ma SL, et al. | March 21, 2019
DIAGNOSTIC CRITERIA:Any one of the following three ,makes it a diagnosis of anphylaxis-
1.Onset within minutes to hours(ACUTE) of generalised itching,hives,swelling of lips,uvula,tounge and flushing(INVOLVEMENT OF SKIN AND/OR MUCOSAL TISSUES) and one of the following two-
a.breathlessness,wheeze,bronchospasm,stridor.hypoxemia,reduced PEF(RESPIRATORY COMPROMISE)
b.Low blood pressure for the age or symptoms of end organ dysfunction in the form of collapse,hypotonia,incontinence or syncope.(HYPOTENSION)
2.Two or more of the following four:
a.Generalised involvement of skin and/or mucosal tissues
b.Respiratory compromise
c.Reduced blood pressure for the age or symptoms of end organ failure
d.Crampy abdominal pain,vomiting which is persistent(PERSISTENT GASTROINTESTINAL SYMPTOMS)
3.Low blood pressure
a. For infants one month to 12 months, systolic BP less than 70mm of Hg and for children 1 year to 10 years Systolic BP less than 70+age in years OR >30% drop in systolic BP
b.For adults ,systolic BP <90mm of Hg OR >30% drop in systolic BP
ESSENTIAL CRITERIA IS ,THE ABOVE FEATURES SHUOLD BE INTERPRETED ONLY AFTER EXPOSURE TO KNOWN OR LIKELY ALLERGEN.
Triggers of anaphylaxis:Foods,medicines,vaccines,immunotherapy,insect venoms,latex,cold exposure and exercise.
IT MAY BE IDIOPATHIC
WHAT HAPPENS IN THE BODY(Pathogenesis): The main process is the release of mediators like histamine and tryptase and cytokines from the cells like mast cell and basophils and possibly from macrophages. These mediators and cytokines produce allergic symptoms in whole body.
The release of these chemicals may be IgE dependent or non IgE dependent.In case of IgE dependent, the child must be exposed previously to an allergen which produces allergen specific antibody(IgE) which gets bound to mast cells. Upon reexposure these mast cells which are bound to IgE start releasing histamines and tryptase which is responsible for the symptoms.
Anaphylaxis may also be caused by release of mediators by the process than IgE mediated like direct release by medication like morphine,by physical factors like cold and exercise,disturbance of leukotriene metabolism like after use of aspirin and non steroidal anti inflammatory drugs,immune aggregates and complement activations like after ithe use of blood products,probably by complement activation like after the use of radiocontrast dyes and IgG mediated reactions like arter the use of humanised monoclonal antibodies and chimeric.
Pathological features are: airway obstruction,pulmonary edema alveolar hemorrhage,visceral congestion ,laryngeal edema and angioedema. Vasomotor dilataton and/or cardiac dysrhythmia is responsible for hypotension (low BP)
INVESTGATIONS: It is a clinical diagnosis.No investgation is reliable,although serum tryptase level may be raised for several hours but not in food allergy.
TREATMENT:The mainstay of treatment is epinephrine(adrenaline).
It comes in the market in injectable form in 1ml ampoule of 1:1000 strength.It shuold be kept at around the temeratue of 25dC.It should be injected undiluted intramuscularly in lateral thigh in the dose of 0.01ml/kg upto a maximum of 0.5ml(1ml=1mg).It can be repeated at an interval of 5 to 15 minutes twice if symptoms persist and intravenous line has not been established.In case of persistent symptoms i.v line should be established and adrenaline drip should be started.For i.v. use the injection must be diluted to 1:10000 strength. In case of non establishment of i.v. line ,it can be given through endotracheal or intraosseous route.
Patient should be kept in supine position with leg end raised in case of hemodynamic compromise.
In case of non response,oxygen inhalation should be started after estalishing airway,N/S or R/L should be given in the dose of 30ml/kg over one hour if BP is low and salbutamol nebulisation should be given if there is bronchspasm.
In case of non responders specially in the users of beta blockres, glucagon should be used and for bronchodilation ipratropium should be used. Some patients may require vasopressin and in case of asystole and pulseless electrical activity atropine should be used.
Other drugs being used are: HI blocker-cetrizine in the dose of 0.25mg/kg upto a maximum of 10 mg orally or Diphenhydramine in the dose of 1.25mg/kg upto a maximum of 50 mg orally or IM,H2blocker-ranitidine and corticosteroids
Crticosteroid-The dose of methyleprednisolone is 1-2mg /kg i.v upto a maximum dose of 125mg.(SOLUMEDROL)
Inramuscular dose is 1mg/kg upto a maximum of 80mg(DEPOMEDROL)
CAUTION:1. Rapid i.v. infusion or incorrect strength during i.v. use of adrenaline may cause pulmonary edema,hypertension and myocardial infarction
2.Rapid i.v. infusion of ranitidine may cause hypotension
Some patients may experience biphasic anaphylaxis. In this phenomenon,the features of anaphylaxix reappears after clinical resolution. In more than 90% of cases,it happens within 4 hours. So patients must be observed for at least 4 hours before discharge.
MANAGEMENT AFTER DEALING WITH EMERGRNCY:Antihistamine should be given for 3 days and it is optional to prescribe 3 days of oral corticosteroid.
REFERENCES:
Sampson, HA, Muñoz-Furlong, A, Campbell, RL. J Allergy Clin Immunol. vol. 117. 2006. pp. 391-7.
(A summary of the second international conference to develop a universally accepted definition of anaphylaxis, establish clinical criteria that would accurately identify cases of anaphylaxis with high precision, and review the evidence on the most appropriate management of anaphylaxis.)
Simons, FE. “Pharmacologic treatment of anaphylaxis: can the evidence base be strengthened”. Curr Opin Allergy Clin Immunol. vol. 10. 2010. pp. 384-93.
Sheikh, A, Shehata, YA, Brown, SG, Simons, FE. “Adrenaline for the treatment of anaphylaxis: Cochrane systematic review”. Allergy. vol. 64. 2009. pp. 204-12.
Sheikh, A, Ten Broek, V, Brown, SG, Simons, FE. “H1-antihistamines for the treatment of anaphylaxis: Cochrane systematic review”. Allergy. vol. 62. 2007. pp. 830-7.
Choo, KJ, Simons, FE, Sheikh, A. “Glucocorticoids for the treatment of anaphylaxis”. Cochrane Database Syst Rev. 2010 Mar 17. pp. CD007596.
Pumphrey, RS. “Lessons for management of anaphylaxis from a study of fatal reactions”. Clin Exp Allergy. vol. 30. 2000. pp. 1144-50.
Cox, L, Nelson, H, Lockey, R. “Allergen immunotherapy: a practice parameter third update”. J Allergy Clin Immunol. vol. 127. 2011. pp. S1-55.
Bock, SA, Muñoz-Furlong, A, Sampson, HA. “Fatalities due to anaphylactic reactions to foods”. J Allergy Clin Immunol. vol. 107. 2001. pp. 191-3.
Bock, SA, Muñoz-Furlong, A, Sampson, HA. “Further fatalities caused by anaphylactic reactions to food, 2001-2006”. J Allergy Clin Immunol. vol. 119. 2007. pp. 1016-8.
Pumphrey, RS, Gowland, MH. “Further fatal allergic reactions to food in the United Kingdom, 1999-2006”. J Allergy Clin Immunol. vol. 119. 2007. pp. 1018-9.
Greenberger, PA, Rotskoff, BD, Lifschultz, B. “Fatal anaphylaxis: postmortem findings and associated comorbid diseases”. Ann Allergy Asthma Immunol. vol. 98. 2007. pp. 252-7.
Simons, FE, Ardusso, LR, Bilò, MB. “International consensus on (ICON) anaphylaxis”. World Allergy Organ J. vol. 7. 2014. pp. 9.
Muraro, A, Roberts, G, Worm, M. “EAACI Food Allergy and Anaphylaxis Guidelines Group. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology”. Allergy. vol. 69. 2014. pp. 1026-45.
Wood, RA, Camargo, CA, Lieberman, P. “Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States”. J Allergy Clin Immunol. vol. 133. 2014. pp. 461-7.
Lieberman, P, Nicklas, RA, Randolph, C. “Anaphylaxis–a practice parameter update 2015”. Ann Allergy Asthma Immunol. vol. 115. 2015 Nov. pp. 341-84.
(This practice parameter provides updated guidelines for the diagnosis and management of anaphylaxis using evidence from recent medical literature.)
Simons, FE, Ebisawa, M, Sanchez-Borges, M. “2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines”. World Allergy Organ J. vol. 8. 2015 Oct 28. pp. 32.
(This provides updated evidence supporting recommendations for the diagnosis and management of anaphylaxis.)
Lee, S, Hess, EP, Lohse, C. “Trends, characteristics, and incidence of anaphylaxis in 2001-2010: A population-based study”. J Allergy Clin Immunol. 2016 Jun 4. pp. S0091-6749.
(These data show an increase in the rate of anaphylaxis between 2001-2010 and describe differences in triggers affecting different age groups.)
Hereditary angioedema is a potential life threatening condition.
It requires earliest possible diagnosis and immediate treatment to save the life in certain cases.
It is caused by either deficiency of C1 inhibitor(Type 1) or dysfunction (Type 2).
It is an autosomal dominant disorder.
In a randomised control trial ,published in JAMA,Lanadelumab,a monoclonal human antibody against Kallikrein was proved to be very effective in reducing the frequency of attacks as well as reducing the severity of individual attack.The level of evidence is level 1,means excellent.
The study was conducted at 41 places in Canada,Jordan,Europe and United states. All the subjects included were previously diagnosed cases of Hereditary angioedema and all were more than 12 years of age.
84 patients were given Lanadelumab and 41 were given placebo.There was a run in period of 4 weeks preceded by 2 weeks of wash out period for any prophylactic treatment being used by patients knowingly or unknowingly. Patients were followed up for a period of 26 weeks for occurence of any episode of angioedema its severity, or any adverse effect of interventional medication.Patients were randomised 2:1 to receive Lanadelumab and placebo. The Lanadelumab receiving patients were divided into 3 groups, and the treatment period was of 26 weeks. The first group received Lanadelumab suncutaneously 150 mg every 4 weeks,second group received 300mg every 4 weeks and the third group 300mg every 2 weeks. The number of episode of angioedema and its severity was observed through a study period of 26 weeks. Adverse events were also observed.
In the group receiving 150mg Lanadelumab subcutaneously every 4 weeks,mean number of attack was 0.48(CI95 0.31-0.73) in comparision to mean number of attack 1.97 in the placebo group(CI95 1.64-2.36) ).In the group receiving 300mg of Lanadelumab every 4 weeks .the mean number of attack was 0.5((CI95 0.36-0.77) ) and for those receiving 300mg of Lanadelumab every 2 weeks the mean number of attack was 0.26(CI95 0.14-0.46) )
Patients receiving Lanadelumab had improved quality of life with reduced number of attacks(p<0.001),less use of anti C1 inhibitor medications( an20.2% vs 65.9%; p < 0.001) and less episode of moderate to severe attacks(p<0.001)
Adverse effects were seen in 98.5% cases and they were mild to moderate, including headace(7.1%),pain at the site of injection(41.7%),erythma at the site of injection(9.5%) and bruising at the site of injection(6%)
REFERENCES:
Pulmonology Advisor > Trial Tracker > Lanadelumab may be an effective prophylactic treatment for hereditary angioedema
Asthma is the most common chronic respiratory disorders in children.
Worldwide,the incidence of asthma has increased over the past few decades.
Mortality from asthma is still high even in this era of sophisticated diagnostic facilities and availabilities of advanced treatment modalities.
If the risk of getting asthmatic is predicted in children,the preventive measures can be applied to some extent.
Hperbilirubinemia is the most common clinical signs warranting treatment in neonates.
The level of serum bilirubin is an indicator of starting phototherapy.
In most of the cases,rise in serum bilirubin is physiological in newborns requiring only reassurance to the parents.
In some newborns,the rise in bilirubin may go unnoticed.
Now,there has been a retrospetive study of asthmatic children,relating the diseases to the level of serum bilirubin during neonatal period.
The study was conducted on 109,212 infants born at or after 35 weeks of gestation. The study centre was Kaiser Permanente Northern California Hospital.Total serum bilirubin was measured over all infants universally at the time of discharge and again at the age of one month.Other covariables were also included in the study like,age,race,ethnicity of mothers and sex,birth weight,gestational age and 5 minutes apgar scores of newborn.
The incidence of asthma,which was the main interest of study was defined as 2 or more visits to OPD with a physician diagnosed asthma and 2 or more asthma medications within a period of one year separeted by a period of one month after the child crosses the age of 2 years..
Among the infants in study group,the highest level of serum bilirubin was 18mg or more in 4.7% and 15mg or more in 16.7%.In 11.5% infants, phototherapy had to be given.
When compared with infants having serum bilirubin between 3-5.9gm/dl,the asthma risk was greater in infants having serum bilirubin level of 9-11.9mg/dl(hazard ratio [HR] 1.22; 95% CI, 1.11-1.34; P <.001),12-14.9mg/dl((HR, 1.18; 95% CI, 1.08-1.29; P <.001) and 15 to 17.9mg/dl(HR, 1.30; 95% CI 1.18-1.43; P <.001)
Interestingly the total serum bilirubin level at or more than 18 mg/dl did not show any correlation with incidence of asthma(HR, 1.04; 95% CI, 0.90-1.20; P =.9)
Induction of phototherapy did not increase the risk of asthma(HR, 1.07; 95% CI, 0.96-1.20).
The causal relationship between asthma and level of serum bilirubin could not be eastablished as the study was limited by its retrospective nature.
REFERENCES:
Kuzniewicz MW, Niki H, Walsh EM, McCulloch CE, Newman TB. Hyperbilirubinemia, phototherapy, and childhood asthma. Pediatrics. 2018;142(4):e20180662.
Congenital hypoventilation syndrome(CCHS) is a rare disorder.
It has myriad clinical presentations,affecting various systems.
It may present at birth or in adulthood depending on mutations.
If it presents in neonatal period,the person becomes ventilator dependent but lator on,may require ventilator only during sleep.
If it presents in adulthood,the person remains asymtomatic during entire childhood period.
Some affected individual becomes symptomatic only during respiratory infections,sedations or during anesthesia due to other surgical reasons.
Some individuals get detected only when there is great difficulties in weaning from ventilator due to any cause.
Some children get detected when they have abnormally high capacity to hold breath for a prolonged period.
Some adults and adolescents have been diagnosed only after they showed abnormally high capacity for a prolonged period to swim underwater.
Some affected individuals may have excessive episodic perspirations(sweating).
Affected individuals may be less sensitive to pain or less anxious in situations where normal individuals have symptoms of anxiety.
There is no sign of respiratory distress even when they are severly hypoxic or hypercapneic.
Following are the clinical manifestations from different systems/Organs:
RESPIRATORY SYSTEM; Alveolar hypoventilation,decreased perception of dyspnea
CARDIOVASCULAR SYSTEM:Increased sinus pause(more than 3 seconds),low day time blood pressure
orthostatic hypotension,bradycardia,less increase in BP after exercise,syncope
NERVOUS SYSTEM:less perception of pain,less anxiety,neurocognitive defect,sizure
GASTROINTESTINAL:Oesophageal dismotility,constipation,in 20%cases may Hirschprungs disease
ENDOCRINE:Hyperglycemia,hypoglycemia,hyperinsulinism
SKIN:Sporadic excessive swaeting
TUMOURS:Neuroblastoma,ganglioneuroma
OTHERS:Low baseline body temperature,heat tolerance may be poor.
CLINICAL PRESENTATIONS:In most of the cases it presents in neonatal period with apnea,hypoventilation of central origin with hypoxemia,hypercapnea and cyanosis.They require assisted ventilation,invasive or non invasive to survive.Once put on ventilator,they are unable to be weaned off from ventilator.As they grow and mature,some require assisted ventilation only during sleep.
Children and adults present with unexplained apnea,respiratory failure during respiratory infections or sedation requiring assisted ventilation.There is no sign of respiratory distress even they are severly hypoxemic or hypercapneic.They may present with unexplained seizure due to undetected hypoxemiaand/or hypercapnea for a prolonged period.
They may show unusual capacity to hold breath for a long time and unusually high capacity for underwater swimming. They may present with features of right heart failure confusing with congenital heart disease.
Older children and adults may present with features of pulmonary hypertension,or cor pulmonale
CAUSES: Respiration is initiated by peripheral chemreceptors located in aortic and carotid bodies, which mainly sense changes in Po2 and to a lesser extent Pco2 and PH ,and central chemreceptors located in medulla, which sense changes in Pc02 and PH.Respiration is controlled by the centre located in medulla and pons which integrate the inputs from chemoreceptors and send signal to the respiratory muscles to perform the process of breathing.
PHOX2B gene, located on chromosome 4p12, is a protein comprising 314 amino acids with two short and stable polyalanine repeats of 9 and 20 residues in the C terminus. It encodes for highly conserved homeodomain transcription factor that is essential in the development of Respiratory control NEURONS and ANS.
CCHS is a result of mutational defect in PHOX2B gene.Normal PHOX2B gene has 20 alanine repeats (20/20 genotype). The majority of CCHS patients have polyalanine repeat mutations(PARM).
Variable clinical manifestations are the results of variable mutations.
DIAGNOSIS: After ruling out other cause of hypoventilations,by approriate investigations,all suspected cases should be confirmed by gene mutatin analysis for PHOX2B gene.There 3 methods available currently.
1.PHOX2B targeted mutation analysis
2.PHOX2B sequence analysis
3. Deletion/duplication test
MANAGEMENT: Goal of management is to provide adequate ventilatory support during both sleep and awake states to maintain pco2 at near to 35mmhg and aeterial saturation above 95%.For asymptomatic patients,it is important to remember that they may go intrespiratory decompensation while in stress,infections or sedations.
As these patients are unable to sense hypoxia as well as hypercarbia,spo2 monitoring and tco2 monitoring particularly during sleep is important.
MODES OF VENTILATORY SUPPORT:Invasive positive pressure ventilation through tracheostomy
Non invasive positive pressure ventilation
Diaphragmatic pacing
GENETIC COUNCELLING:PHOX2B gene mutation has autosomal pattern of inheritance with variable penetrance.
Genetic counseling is important as there is 50% chance of recurrence with each
child.So if a parent is affected,there is 50% chance for siblings to get
affected
It is important to test parents of affected child even if they are asymptomatic.
References
1. Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG,
Loghmanee DA, Trang H. An official ATS clinical policy statement:
Congenital central hypoventilation syndrome: genetic basis, diagnosis,
and management. Am J Respir Crit Care Med. 2010;181(6):626–644.
2. Fleming PJ, Cade D, Bryan MH, Bryan AC. Congenital central
hypoventilation and sleep state. Pediatrics. 1980;66(3):425–428.
3. Huang J, Colrain IM, Panitch HB, et al. Effect of sleep stage on
breathing in children with central hypoventilation. J Appl Physiol.
2008;105(1):44–53.
4. Chen ML, Keens TG. Congenital central hypoventilation syndrome:
not just another rare disorder. Paediatr Respir Rev. 2004;5(3):182–189.
5. Weese-Mayer DE, Shannon DC, Keens TG, Silvestri JM. Idiopathic
congenital central hypoventilation syndrome: diagnosis and management.
American Thoracic Society. Am J Respir Crit Care Med.
1999;160(1):368–373.
6. Trang H, Dehan M, Beaufils F, Zaccaria I, Amiel J, Gaultier C. The
French Congenital Central Hypoventilation Syndrome Registry:
general data, phenotype, and genotype. Chest. 2005;127(1):72–79.
7. Shimokaze T, Sasaki A, Meguro T, et al. Genotype–phenotype relationship
in Japanese patients with congenital central hypoventilation
syndrome. J Hum Genet. 2015;60(9):473–477.
8. Amiel J, Laudier B, Attié-Bitach T, et al. Polyalanine expansion and
frameshift mutations of the paired-like homeobox gene PHOX2B
in congenital central hypoventilation syndrome. Nat Genet.
2003;33(4):459–461.
9. Weese-Mayer DE, Berry-Kravis EM, Zhou L, et al. Idiopathic
congenital central hypoventilation syndrome: Analysis of genes
pertinent to early autonomic nervous system embryologic development
and identification of mutations in PHOX2b. Am J Med Genet.
2003;123A(3):267–278.
10. Hasegawa H, Kawasaki K, Inoue H, Umehara M, Takase M. Japanese
Society of Pediatric Pulmonology Working Group (JSPPWG). Epidemiologic
survey of patients with congenital central hypoventilation
syndrome in Japan. Pediatr Int. 2012;54(1):123–126.
11. Kasi A, Perez I, Kun S, Keens T. Congenital central hypoventilation
syndrome: Diagnostic and management challenges. Pediatr Heal Med
Ther. 2016;7:99–107.
12. Maloney MA, Kun SS, Keens TG, Perez IA. Congenital central
hypoventilation syndrome: diagnosis and management. Expert Rev
Respir Med. 2018;12(4):283–292.
13. Kasi AS, Jurgensen TJ, Yen S, et al. Three-Generation Family
With Congenital Central Hypoventilation Syndrome and Novel
PHOX2B Gene Non-Polyalanine Repeat Mutation. J Clin Sleep Med.
2017;13(07):925–927.
14. Vanderlaan M, Holbrook CR, Wang M, Tuell A, Gozal D. Epidemiologic
survey of 196 patients with congenital central hypoventilation
syndrome. Pediatr Pulmonol. 2004;37(3):217–229.
15. Magalhães J, Madureira N, Medeiros R, et al. Late-onset congenital
central hypoventilation syndrome and a rare PHOX2B gene mutation.
Sleep Breath. 2015;19(1):55–60.
16. Doherty LS, Kiely JL, Deegan PC, et al. Late-onset central hypoventilation
syndrome: a family genetic study. Eur Respir J. 2007;29(2):312–316.
17. Barratt S, Kendrick AH, Buchanan F, Whittle AT. Central hypoventilation
with PHOX2B expansion mutation presenting in adulthood.
Thorax. 2007;62(10):919–920.
18. Trang H, Laudier B, Trochet D, et al. PHOX2B gene mutation in a
patient with late-onset central hypoventilation. Pediatr Pulmonol.
2004;38(4):349–351.
19. Mahmoud M, Bryan Y, Gunter J, Kreeger RN, Sadhasivam S. Anesthetic
implications of undiagnosed late onset central hypoventilation
syndrome in a child: from elective tonsillectomy to tracheostomy.
Pediatric Anesthesia. 2007;17(10):1001–1005.
20. Mahfouz AK, Rashid M, Khan MS, Reddy P. Late onset congenital
central hypoventilation syndrome after exposure to general anesthesia.
Can J Anaesth. 2011;58(12):1105–1109.
21. Antic NA, Malow BA, Lange N, et al. PHOX2B mutation-confirmed
congenital central hypoventilation syndrome: Presentation in adulthood.
Am J Respir Crit Care Med. 2006;174(8):923–927.
22. Repetto GM, Corrales RJ, Abara SG, et al. Later-onset congenital
central hypoventilation syndrome due to a heterozygous 24-polyalanine
repeat expansion mutation in the PHOX2B gene. Acta Paediatr.
2009;98(1):192–195.
23. Paton JY, Swaminathan S, Sargent CW, Keens TG. Hypoxic and
hypercapnic ventilatory responses in awake children with congenital
central hypoventilation syndrome. Am Rev Respir Dis.
1989;140(2):368–372.
24. Shea SA, Andres LP, Paydarfar D, Banzett RB, Shannon DC. Effect
of mental activity on breathing in congenital central hypoventilation
syndrome. Respir Physiol. 1993;94(3):251–263.
25. Diedrich A, Malow BA, Antic NA, et al. Vagal and sympathetic heart
rate and blood pressure control in adult onset PHOX2B mutationconfirmed
congenital central hypoventilation syndrome. Clin Auton
Res. 2007;17(3):177–185.
26. Rita Azeredo Bittencourt L, Pedrazzoli M, Yagihara F, et al. Lateonset,
insidious course and invasive treatment of congenital central
hypoventilation syndrome in a case with the Phox2B mutation: case
report. Sleep Breathe. 2012;16:951–955.
27. Antic N, Mcevoy RD. Primary alveolar hypoventilation and
response to the respiratory stimulant almitrine. Intern Med J.
2002;32(12):622–624.
28. F-G MR, Manna S, Durward A. Cor pulmonale due to congenital
central hypoventilation syndrome presenting in adolescence. Pediatr
Crit Care Med. 2009;10(4):e41-2 1p.
29. Chuen-Im P, Marwan S, Carter J, Kemp J, Rivera-Spoljaric K.
Heterozygous 24-polyalanine repeats in the PHOX2B gene with
different manifestations across three generations. Pediatr Pulmonol.
2014;49(2):E13–E16.
30. Swaminathan S, Gilsanz V, Atkinson J, Thomas GK, Keens TG. Congenital
central hypoventilation syndrome associated with multiple
ganglioneuromas. Chest. 1989;96(2):423–424.
31. Jennings LJ, Yu M, Rand CM, et al. Variable human phenotype associated
with novel deletions of the PHOX2B gene. Pediatr Pulmonol.
2012;47(2):153–161.
32. Trochet D, O’Brien LM, Gozal D, et al. PHOX2B genotype allows
for prediction of tumor risk in congenital central hypoventilation
syndrome. Am J Hum Genet. 2005;76(3):421–426.
33. Faure C, Viarme F, Cargill G, Navarro J, Gaultier C, Trang H. Abnormal
esophageal motility in children with congenital central hypoventilation
syndrome. Gastroenterology. 2002;122(5):1258–1263.
34. Rohrer T, Trachsel D, Engelcke G, Hammer J, Hammer J. Congenital
central hypoventilation syndrome associated with Hirschsprung’s disease
and neuroblastoma: case of multiple neurocristopathies. Pediatr
Pulmonol. 2002;33(1):71–76.
35. Woo MS, Woo MA, Gozal D, Jansen MT, Keens TG, Harper RM.
Heart rate variability in congenital central hypoventilation syndrome.
Pediatr Res. 1992;31(3):291–296.
36. Silvestri JM, Hanna BD, Volgman AS, Jones PJ, Barnes SD, Weese-
Mayer DE. Cardiac rhythm disturbances among children with idiopathic
congenital central hypoventilation syndrome. Pediatr Pulmonol.
2000;29(5):351–358.
37. Gronli JO, Santucci BA, Leurgans SE, Berry-Kravis EM, Weese-
Mayer DE. Congenital central hypoventilation syndrome:PHOX2B
genotype determines risk for sudden death. Pediatr Pulmonol.
2008;43(1):77–86.
38. Trang H, Girard A, Laude D, Elghozi JL. Short-term blood pressure and
heart rate variability in congenital central hypoventilation syndrome
(Ondine’s curse). Clin Sci. 2005;108(3):225–230.
39. Trang H, Boureghda S, Denjoy I, Alia M, Kabaker M. 24-hour BP
in children with congenital central hypoventilation syndrome. Chest.
2003;124(4):1393–1399.
40. Goldberg DS, Ludwig IH. Congenital central hypoventilation syndrome:
ocular findings in 37 children. J Pediatr Ophthalmol Strabismus.
1996;33(3):175–180.
41. Basu AP, Bellis P, Whittaker RG, Mckean MC, Devlin AM. Teaching
NeuroImages: Alternating ptosis and Marcus Gunn jaw-winking
phenomenon with PHOX2B mutation. Neurology. 2012;79(17):e153.
42. Patwari PP, Stewart TM, Rand CM, et al. Pupillometry in congenital
central hypoventilation syndrome (CCHS): quantitative
evidence of autonomic nervous system dysregulation. Pediatr Res.
2012;71(3):280–285.
43. Boulanger-Scemama E, Fardeau C, Straus C, et al. Ophthalmologic
impairment during adulthood in central congenital hypoventilation
syndrome: a longitudinal cohort analysis of nine patients. Ophthalmic
Genet. 2014;35(4):229–234.
44. Hennewig U, Hadzik B, Vogel M, et al. Congenital central hypoventilation
syndrome with hyperinsulinism in a preterm infant. J Hum Genet.
2008;53(6):573–577.
45. Farina MI, Scarani R, Po’ C, Agosto C, Ottonello G, Benini F. Congenital
central hypoventilation syndrome and hypoglycaemia. Acta
Paediatr. 2012;101(2):e92–e96.
46. Marics G, Amiel J, Vatai B, Lódi C, Mikos B, Tóth-Heyn P. Autonomic
dysfunction of glucose homoeostasis in congenital central hypoventilation
syndrome. Acta Paediatr. 2013;102(4):e178–e180.
47. Gelwane G, Trang H, Carel J-C, Dauger S, Léger J. Intermittent
hyperglycemia due to autonomic nervous system dysfunction: a new
feature in patients with congenital central hypoventilation syndrome.
J Pediatr. 2013;162(1):171–176.
48. Hopkins E, Stark J, Mosquera RA. Central Congenital Hypoventilation
Syndrome associated with hypoglycemia and seizure. Respir Med Case
Rep. 2017;20:75–76.
49. Saiyed R, Rand CM, Carroll MS, et al. Congenital central hypoventilation
syndrome (CCHS): Circadian temperature variation. Pediatr
Pulmonol. 2016;51(3):300–307.
50. Weese-Mayer DE, Rand CM, Zhou A, Carroll MS, Hunt CE. Congenital
central hypoventilation syndrome: a bedside-to-bench success story
for advancing early diagnosis and treatment and improved survival and
quality of life. Pediatr Res. 2017;81(1-2):192–201.
51. Pattyn A, Morin X, Cremer H, Goridis C, Brunet J-F. The homeobox
gene Phox2b is essential for the development of autonomic neural
crest derivatives. Nature. 1999;399(6734):366–370.
52. Pattyn A, Morin X, Cremer H, Goridis C, Brunet J-F. Expression and interactions
of the two closely related homeobox genes Phox2a and Phox2b
during neurogenesis. Development. 1997;1249374403(20):4065-4075.
53. Marcus CL, Bautista DB, Amihyia A, Ward SL, Keens TG. Hypercapneic
arousal responses in children with congenital central hypoventilation
syndrome. Pediatrics. 1991;88(5):993–998.
54. Gozal D, Marcus CL, Shoseyov D, Keens TG. Peripheral chemoreceptor
function in children with the congenital central hypoventilation
syndrome. J Appl Physiol. 1993;74(1):379–387.
55. Stornetta RL, Moreira TS, Takakura AC, et al. Expression of Phox2b
by brainstem neurons involved in chemosensory integration in the
adult rat. J Neurosci. 2006;26(40):10305–10314.
56. Takakura AC, Barna BF, Cruz JC, Colombari E, Moreira TS. Phox2bexpressing
retrotrapezoid neurons and the integration of central and
peripheral chemosensory control of breathing in conscious rats. Exp
Physiol. 2014;99(3):571–585.
57. Dauger S. Phox2b controls the development of peripheral chemoreceptors
and afferent visceral pathways. Development.
2003;130(26):6635–6642.
58. Moreira TS, Takakura AC, Czeisler C, Otero JJ. Respiratory and autonomic
dysfunction in congenital central hypoventilation syndrome. J
Neurophysiol. 2016;116(2):742–752.
59. Guyenet PG, Stornetta RL, Abbott SBG, Depuy SD, Kanbar R. The
retrotrapezoid nucleus and breathing. In: Advances in Experimental
Medicine and Biology. Vol. 2012;758:115–122.
60. Dubreuil V, Barhanin J, Goridis C, Brunet J-F. Breathing with Phox2b.
Philosophical Transactions of the Royal Society B: Biological Sciences.
2009;364(1529):2477–2483.
61. Dubreuil V, Ramanantsoa N, Trochet D, et al. A human mutation in
Phox2b causes lack of CO2 chemosensitivity, fatal central apnea,
and specific loss of parafacial neurons. Proc Natl Acad Sci U S A.
2008;105(3):1067–1072.
62. Rudzinski E, Kapur RP. PHOX2B immunolocalization of the
candidate human retrotrapezoid nucleus. Pediatr Dev Pathol.
2010;13(4):291–299.
63. Pattyn A, Goridis C, Brunet JF. Specification of the central noradrenergic
phenotype by the homeobox gene Phox2b. Mol Cell Neurosci.
2000;15(3):235–243.
64. Nobuta H, Cilio MR, Danhaive O, et al. Dysregulation of locus coeruleus
development in congenital central hypoventilation syndrome.
Acta Neuropathol. 2015;130(2):171–183.
65. Tomycz ND, Haynes RL, Schmidt EF, Ackerson K, Kinney HC. Novel
neuropathologic findings in the Haddad syndrome. Acta Neuropathol.
2010;119(2):261–269.
66. Ogren JA, Macey PM, Kumar R, Woo MA, Harper RM. Central
autonomic regulation in congenital central hypoventilation syndrome.
Neuroscience. 2010;167(4):1249–1256.
67. Weese-Mayer DE, Marazita ML, Rand CM, Berry-Kravis EM.
Congenital Central Hypoventilation Syndrome. Seattle: University
of Washington; 2014. Available from: http://www.ncbi.nlm.nih.gov/
pubmed/20301600. Accessed July 25, 2018.
68. Jennings LJ, Yu M, Zhou L, Rand CM, Berry-Kravis EM, Weese-
Mayer DE. Comparison of PHOX2B testing methods in the diagnosis
of congenital central hypoventilation syndrome and mosaic carriers.
Diagn Mol Pathol. 2010;19(4):224–231.
69. Bachetti T, Parodi S, di Duca M, Santamaria G, Ravazzolo R, Ceccherini
I. Low amounts of PHOX2B expanded alleles in asymptomatic
parents suggest unsuspected recurrence risk in congenital central
hypoventilation syndrome. J Mol Med. 2011;89(5):505–513.
70. Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour
Holter Monitoring with 14-day Novel Adhesive Patch Electrocardiographic
Monitoring. Am J Med. 2014;127(1):95.e11-95.e17–95.
71. Walsh JA, Topol EJ, Steinhubl SR. Novel Wireless Devices for Cardiac
Monitoring. Circulation. 2014;130(7):573–581.
72. Zelko FA, Nelson MN, Leurgans SE, Berry-Kravis EM, Weese-Mayer
DE. Congenital central hypoventilation syndrome: Neurocognitive functioning
in school age children. Pediatr Pulmonol. 2010;45(1):92–98.
73. Charnay AJ, Antisdel-Lomaglio JE, Zelko FA, et al. Congenital Central
Hypoventilation Syndrome: Neurocognition Already Reduced in
Preschool-Age Children. Chest. 2015.
74. Silvestri JM, Weese-Mayer DE, Nelson MN. Neuropsychologic abnormalities
in children with congenital central hypoventilation syndrome.
J Pediatr. 1992;120(3):388–393.
75. Zelko FA, Stewart TM, Brogadir CD, Rand CM, Weese-Mayer DE.
Congenital central hypoventilation syndrome: Broader cognitive deficits
revealed by parent controls. Pediatr Pulmonol. 2018;53(4):492–497.
76. Marcus CL, Jansen MT, Poulsen MK, et al. Medical and psychosocial
outcome of children with congenital central hypoventilation syndrome.
J Pediatr. 1991;119(6):888–895.
77. Diep B, Wang A, Kun S, et al. Diaphragm Pacing without Tracheostomy
in Congenital Central Hypoventilation Syndrome Patients. Respiration.
2015;89(6):534–538.
78. Matera Iet al. PHOX2B mutations and polyalanine expansions correlate
with the severity of the respiratory phenotype and associated
symptoms in both congenital and late onset Central Hypoventilation
syndrome. J Med Genet. 2004;41(5):373–380.
79. Trochet D, Hong SJ, Lim JK, et al. Molecular consequences of PHOX2B
missense, frameshift and alanine expansion mutations leading to autonomic
dysfunction. Hum Mol Genet. 2005;14(23):3697–3708.
80. Bachetti T, Matera I, Borghini S, Duca MD, Ravazzolo R, Ceccherini
I. Distinct pathogenetic mechanisms for PHOX2B associated polyalanine
expansions and frameshift mutations in congenital central
hypoventilation syndrome. Hum Mol Genet. 2005;14(13):1815–1824.
81. di Lascio S, Bachetti T, Saba E, Ceccherini I, Benfante R, Fornasari
D. Transcriptional dysregulation and impairment of PHOX2B autoregulatory
mechanism induced by polyalanine expansion mutations
associated with congenital central hypoventilation syndrome. Neurobiol
Dis. 2013;50:187–200.
82. H-T W, Y-N S, Hung C-C, Hsieh W-S, K-J W. Interaction between
PHOX2B and CREBBP mediates synergistic activation: mechanistic
implications of PHOX2B mutants. Hum Mutat. 2009;30(4):655–660.
83. Low KJ, Turnbull AR, Smith KR, et al. A case of congenital central hypoventilation
syndrome in a three-generation family with non-polyalanine repeat
PHOX2B mutation. Pediatr Pulmonol. 2014;49(10):E140–E143.
84. Cain JT, Kim DI, Quast M, et al. Nonsense pathogenic variants in exon
1 of PHOX2B lead to translational reinitiation in congenital central
hypoventilation syndrome. Am J Med Genet A. 2017;173(5):1200–1207.
85. Lombardo RC, Kramer E, Cnota JF, Sawnani H, Hopkin RJ. Variable
phenotype in a novel mutation in PHOX2B. Am J Med Genet A.
2017;173(6):1705–1709.
86. di Lascio S, Benfante R, di Zanni E, et al. Structural and functional
differences in PHOX2B frameshift mutations underlie isolated or
syndromic congenital central hypoventilation syndrome. Hum Mutat.
2018;39(2):219–236.
87. Berry-Kravis EM, Zhou L, Rand CM, Weese-Mayer DE. Congenital
central hypoventilation syndrome: PHOX2B mutations and phenotype.
Am J Respir Crit Care Med. 2006;174(10):1139–1144.
88. Kwon M-J, Lee G-H, Lee M-K, et al. PHOX2B mutations in patients
with Ondine–Hirschsprung disease and a review of the literature. Eur
J Pediatr. 2011;170(10):1267–1271.
89. Trang H, Brunet J-F, Rohrer H, et al. Proceedings of the fourth international
conference on central hypoventilation. Orphanet J Rare Dis.
2014;9(1):194.
90. Beckerman R. Home positive pressure ventilation in congenital central
hypoventilation syndrome: more than twenty years of experience.
Pediatr Pulmonol. 1997;23:154–155.
91. Perez IA, Keens TG, Davidson Ward SL. Noninvasive positive pressure
ventilation in the treatment of hypoventilation in children. Sleep Med
Clin. 2010;5(3):471–484.
92. Kerbl R, Litscher H, Grubbauer HM, et al. Congenital central
hypoventilation syndrome (Ondine’s curse syndrome) in two siblings:
Delayed diagnosis and successful noninvasive treatment. Eur J Pediatr.
1996;155(11):977–980.
93. Migliori C, Cavazza A, Motta M, Bottino R, Chirico G. Early use of
Nasal-BiPAP in two infants with Congenital Central Hypoventilation
syndrome. Acta Paediatr. 2003;92(7):823–826.
94. Vagiakis E, Koutsourelakis I, Perraki E, et al. Average volume-assured
pressure support in a 16-year-old girl with congenital central hypoventilation
syndrome. J Clin Sleep Med. 2010;6(6):609–612.
95. Kam K, Bjornson C, Mitchell I. Congenital central hypoventilation
syndrome; Safety of early transition to non-invasive ventilation. Pediatr
Pulmonol. 2014;49(4):410–413.
96. Khayat A, Medin D, Syed F, et al. Intelligent volume-assured pressured
support (iVAPS) for the treatment of congenital central hypoventilation
syndrome. Sleep Breath. 2017;21(2):513–519.
97. Weese-Mayer DE, Hunt CE, Brouillette RT, Silvestri JM. Diaphragm
pacing in infants and children. J Pediatr. 1992;120(1):1–8.
98. Chen ML, Tablizo MA, Kun S, Keens TG. Diaphragm pacers as a
treatment for congenital central hypoventilation syndrome. Expert
Rev Med Devices. 2005;2(5):577–585.
99. Hartmann H, Jawad MH, Noyes J, Samuels MP, Southall DP. Negative
extrathoracic pressure ventilation in central hypoventilation syndrome.
Arch Dis Child. 1994;70(5):418–423.
100. Tibballs J, Henning RD. Noninvasive ventilatory strategies in
the management of a newborn infant and three children with
congenital central hypoventilation syndrome. Pediatr Pulmonol.
2003;36(6):544–548.
101. Shaul DB, Danielson PD, Mccomb JG, Keens TG. Thoracoscopic
placement of phrenic nerve electrodes for diaphragmatic pacing in
children. J Pediatr Surg. 2002;37(7):974–978.
102. Nicholson KJ, Nosanov LB, Bowen KA, et al. Thoracoscopic placement
of phrenic nerve pacers for diaphragm pacing in congenital central
hypoventilation syndrome. J Pediatr Surg. 2015;50(1):78–81.
103. Wang A, Kun S, Diep B, Davidson Ward SL, Keens TG, Perez
IA. Obstructive Sleep Apnea in Patients With Congenital Central
Hypoventilation Syndrome Ventilated by Diaphragm Pacing Without
Tracheostomy. J Clin Sleep Med. 2018;14(2):261–264.
104. Sritippayawan S, Hamutcu R, Kun SS, Ner Z, Ponce M, Keens TG.
Mother-daughter transmission of congenital central hypoventilation
syndrome. Am J Respir Crit Care Med. 2002;166(3):367–369.
105. Rajendran GP, Kessler MS, Manning FA. Congenital Central Hypoventilation
syndrome (Ondine’s curse): prenatal diagnosis and fetal breathing
characteristics. J Perinatol. 2009;29(10):712–713
Salbutomol/Levosalbutamol is the main drug for rescue in acute exacerbation of asthma.
Most of the asthmatic patients respond very well when this drug is given through metered dose inhaler(MDI)
Very few patients require this drug to be given through nebuliser.
Some patients may not respond to salbutamol/levosalbutamol due to mutation of beta 2 receptor,making this drug partially responsive or non responsive.
Every patient with asthma, whether mild,moderate,or severe are prescribed salbutamol/levosalbutamol for use when they feel symptoms of asthma.
Patients on regular controller medications are advised to take salbutamol/levosalbutamol through MDI ,when they perceive the symptom of asthma.
Few patients complaint of getting their symptoms worse after taking salbutamol/levosalbutamol instead of getting better. In usual circumstances,these complaints are ignored by treating physicians.This is a phenomenon of paradoxical bronchospasm.Here is a case report.
A 25 years old African American suffering from moderate asthma with allergic rhinitis came for assessment including Pulmonary function test(PFT) in the clinic.He was using salmetrol with fluticasone,monteleukast and salbutamol for last 4 weeks. He was complaining of increased shortness of breath after taking salbutamol hydrofluoroalkane(HFA) through metered dose inhaler(MDI).
In the clinic, PFT was performed before and after giving 4 puffs of levosalbutamol. FVC decreased from 4.17 L(76%Predicted) to 4.07L(74% predicted). FEV1 decreased from 2.19L(50%predicted) to 1.70(39%predicted).
Similarly the FVCdecreased from 4.99L(107%predicted) to 4.42L(95%predicted) and FEV1 decreased from 2.68(68%) to 2.25 (57%) after administration of 4 puffs of Salbutamol.
When the salbutamol was given through nebuliser in the dose of 2.5mg, there was an increase in FEV1,while no change in FVC was noticed.
After seeing such response,in contrary to the expectation,the PFT was performed before and after giving 4 puffs of ipratorium bromide to asseess the efficacy of this drug in acute exacerbation of asthma. There was no change in FVC 4.85L (104%),while the FEV1 increased from 2.47L(62%) to 2.73L(69%).
After the assessment and PFTs in clinic ,the patient was prescribed Ipratropium bromide HFA,MDI for rescue therapy and the dose of fluticasone/salmetrol was increased. Salbutamol MDI was withdrawn from the treatment. The patient is not reporting symptoms after this transition was made.
As the patient was feeling uncomfortable after salbutamol MDI,while getting better with the same drug through nubuliser,it was suspected that the inactive ingredient in inlaher was responsible for the bronchospasm.
So the paradoxical bronchospasm should be kept in mind as an adverse effect ,while prescribing salbutamol/levosalbutamol as a rscue medications to asthmatic patients.
REFERENCES:
Magee JS, Pittman LM, Jette-Kelly LA. Paraxdoxical bronchoconstriction with short-acting beta agonist. Am J Case Rep. 2018;19:1204-1207. doi:10.12659/AJCR.910888.
Influenza may be seasonal during cold weather or throughtout the year as in India.
In India the highest number of cases are usually seen during rainy season.
There are three types of influenza viruses,Influenza A,B and C.
Influenza C virus usually causes mild and sporadic disease.
The particular strain H1N1 is called swine flu.
A person suffering from influenza can infect others , 24 hours before the onset of symptoms to 5 days after disappearance of symptoms.
Young children can shed virus for a longer time than adults and elderly.
Immunocompromised individual can shed virus for weeks to months.
The symptoms can appear very fast within a day or may progress overs days.
The incubation period is 1-4 days.
Immunised persons can have mild symptoms even if they get infected.
In mostof the caese ,the disease gets resolved in 3-7 days.
In few cases ,the generalised malaise and cough persist for 2 weeks or more.
Children particularly below 5 years of age, have more complications,more rates of hospitalisations,and more rates of deaths in comparision to adults.
Immunocompromised individuals may have severe disease and high chance of death due to influenza ,so they are particularly advised to get immunised.
The disease spreads through droplets from person to person, formed during coughing or sneezing.
SIGNS AND SYMPTOMS:
Running nose is less common in influenza.
Patients or Parents complaint of headache,fever which may range from 100.4dF to 104dF.
Generalised bodyache,malaise,fatigueness.
No interest in eating ,playing or working depending on age.
Pain in throat and limb pain which makes children unable to walk.
Child may suffer from diarrhoea.
Cough may be troublesome which is usually dry cough but sputum may be produced.
Nausea and vomoting are common in children.
Blocked nose may be a complaint
Chest pain may be a complaint due to pleuritis.
On examination:
child may be dull
Eyes may be red and watery
Throat may be red due to pharyngitis
There may be cervical lymphadenopathy
On chest auscultation,there may be bilateral diffuse wheezes and crackles. If the finding is localised, it indicates secondary bacterial infection.
In severe cases,there may be signs of respiratory failure.
There may be generalised muscular tenderness.
There may be signs of dehydration.
Investigations:The gold standard investigation for confirming the diagnosis is RT-PCR or culture of nasopharyngeal or Pharyngeal swab.
Chest X-Ray shows bilateral diffuse reticular opacities or bilateral diffuse patchy opacities.
If the opacity is in lobar pattern,it indicates secondary bacterial infection.
Chest X-Ray may be normal.
Complete blood count is usually normal.
TREATMENT:Treatment is usually symptomatic. Paracetamol is given for pain and fever.
Salicylate should not be given due to fear of Rye syndrome.
Neuraminidase inhibitor is the drug of choice when the disease is severe enough to be treated by antiviral.
It acts against both A and B influenza.OSELTAMIVIR is recommended for infants and children
IT SHOUD BE STARTED WITHIN 48 HOURS OF ONSET OF SYMPTOMS
DOSES: For preterm neonates-1mg/kg/dose bid ,oral
For full term neonates:
less than 14 days old 3mg/kg/dose, once daily for 5 days,oral
More than 14 days old 3mg/kg/dose, bid for 5 days,oral
INFANTS :
LESS THAN 3 MONTHS:12mg,po,bid for 5 days
3-5 months:20mg po bid for 5 days
6-11months:25 mg po bid for 5 days
CHILDREN:
Less than 5 kg weight-30mg,po,bid for 5 days
5-23 kg-45mg,po,bid for 5 days
23-40kg-60mg,po,bid for 5 days
more than 40 kg -75mg,po,bid for 5 days
ORAL SUSPENSION CONTAINS 6 mg/ml
IMMUNISATION: It is recommended for all children above 6 months of age.
Below 6 months of age they are protected by antibody from mother.
Individuals having chronic lung,liver,kidney disease and primary or secondary
immunodeficiencies are particularly advised for vaccination.
There are two types of vaccines-Live attenuated and inactivated.
Live attenuated is recommended above 2 years of age and it is given intranasally.
Inactivated vaccines are either trivalent or tetravalent.
Tetravalent have better protection for influenza B along with A.
The vaccine is updated every year as the virus changes itself rapidly due to antigenic shift and drift
property.
It should be given as early as it becomes availabe and definitely before the start of season.
It takes 10-14 days for the start of protection after vaccination.
The inactivated vaccine is given intramuscularly,0.25ml for children between 3-36 months and
0.5ml for children above 36 months.
For the first time recipient, it is given in two doses ,4 weeks apart and then single dose every year.
REFERENCES:
;